Vanguard Cleaning Systems
Medical Device Facility Cleaning

Medical Device Manufacturers BIGGEST CHALLENGE
Of the numerous challenges facing medical device manufacturers, the most cited are controlling production and launch costs.
Increased federal regulation and customer expectation, combined with a 2.3% device tax have taken a toll on an emerging and competitive market.
Recent headlines have proven that price hikes on medication and medical devices can result in a financially crippling public backlash.
From a business perspective, one of the primary avenues toward reducing overhead is to standardize and regulate business processes.
This allows day-to-day operations to run smoothly, with minimal oversight, freeing up critical resources and personnel to focus on profit-forward projects.
Green medical cleaning services should play a vital role in reducing operational costs while improving time to market launches, as well as eliminating potential issues with federally mandated cleanliness and sterilization requirements.
In This Article
The Role of Green Cleaning Services
How clean do medical device manufacturing plants need to be?
To control desirable levels of surface contamination, conventional cleaning methods must be ruled out for green medical cleaning services best practices.
As an example; one of the chief concerns facing medical device manufacturers, which green practices address, is cross-contamination, which is easily managed with color-coded microfiber devices.
Additionally, operational cost-reduction goals can be achieved by eliminating natural resource waste (water and electricity), and reducing expenses associated with contaminant waste disposal (green cleaning products are non-toxic and eco-friendly).
The use of non-toxic cleaning solutions will eliminate the potential for toxic residue transferring to, and contaminating, medical devices.
Medical Device Contamination
Contamination classifications are broken up into risk categories based on the nature and duration of device contact with humans.
In essence; the longer the duration and the more invasive the nature of the device, the higher the risk.
The higher the risk factor, the more attention needs to be focused on certified device and surface sterilization.
Certified device sterilization ensures the surface of medical equipment is within manufacturer prescribed residue tolerance levels that are considered safe for humans.
Surface sterilization prevents the transfer of residue to the device after it has been cleaned.
Financial Consequences of Contaminated Medical Devices
In May of 2002 Sulzer Orthopedics of Austin, Texas settled a class action lawsuit against them for approximately $1 Billion.
The class action suit came on the heels of a hip replacement implant recall of 31,000 devices, 17,500 of which had already been implanted.
The decision that led to this disaster was derived from a need to cut production costs, a decision that ultimately led to Sulzer changing its name in October of 2002 to Centerpulse Orthopedics.
A more advisable alternative would be to implement cost-effective green cleaning practices throughout the facility.
Cost-Effective Surface Sterilization With Microfiber
Sterilization requirements vary based on risk-factor, e.g., blood-pressure cuffs are not held to the same standards as dialysis equipment or grafts.
Standard residue classifications are;
- Inorganic – Metals and ceramics, often used in electronic devices.
- Organic – Specifically, residue left over from detergents or other cleaning materials, and;
- Biological – Bacteria and virus’.
Medical grade microfiber cloths address all three classification requirements without the need for toxic emulsifiers or dangerous antibacterial cleaners.
The pockets formed in the creation of microfiber threads have been shown to draw in dirt and harmful bacteria with nothing more than water as an emulsifier.
The pockets are especially efficient at removing metal particles from surfaces, preventing potentially deadly cross-contamination.
The Efficacy of Microfiber in Hospitals
Studies have proven the efficacy of microfiber cloths over conventional, cotton and detergent based cleaning systems, at dirt and bacteria removal.
According to the U.S. Centers for Disease Control and Prevention (CDC);
[sic]…the microfiber system tested demonstrated superior microbial removal compared with conventional string mops when used with a detergent cleaner (94% vs 68%). The use of a disinfectant did not improve the microbial elimination demonstrated by the microfiber system (95% vs 94%). However, use of disinfectant significantly improved microbial removal when a conventional string mop was used (95% vs 68%)…[sic]. The microfiber system also prevents the possibility of transferring microbes from room to room because a new microfiber pad is used in each room.
–Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008
Microfiber as a Mechanism to Reduce Operational Overhead in Medical Cleaning Services
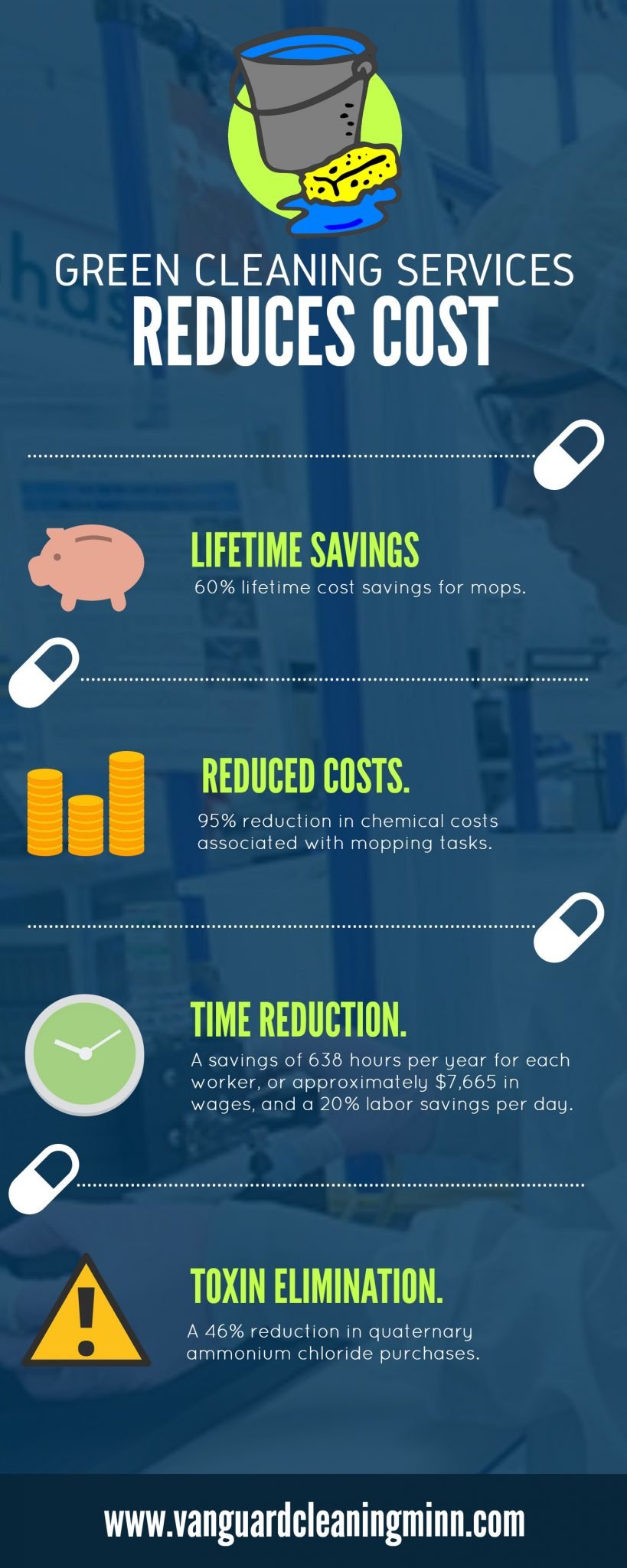
In 1999, the U.C. Davis Medical Center began a pilot program to test the efficacy and financial viability of microfiber use in a hospital as a potential replacement for established cleaning products and protocols.
Highlights of the program included;
- 60% lifetime cost savings for mops.
- 95% reduction in chemical costs associated with mopping tasks.
- 20% labor savings per day.
- A savings of 638 hours per year for each worker, or approximately $7,665 in wages, and;
- A 46% reduction in quaternary ammonium chloride purchases.
For More Information:
- Using Microfiber Mops in Hospitals
References
- Siemens – Top Five Challenges to Medical Device Manufacturers
- Medical Device and Diagnostic Industry – Lean Manufacturing and Sustainability for Medical Device Manufacturers
Takeaway
To ethically cut production costs while maintaining increasing federally required sanitation standards, medical device manufacturers must implement green medical cleaning services, practices, and policies across their entire operation.
To facilitate a seamless transition that requires minimal supervision, as well as fluctuating need requirements, it is imperative the manufacturers partner with a provider possessing a proven track record with the industry.
Search Our Blog
Subscribe to Our Blog
TAKE ACTION
Worried about your medical facility being cross-contaminated due to poor cleaning practices? Maybe it’s time to hire an experienced contract cleaning company to partner with the staff.
Vanguard Cleaning Systems of Minnesota can help you consider all your needs and options before you make a move. Download our free guide, “8 Steps to Hiring a Commercial Cleaning Company”

